Home
Products
TAE684 (NVP-TAE684)
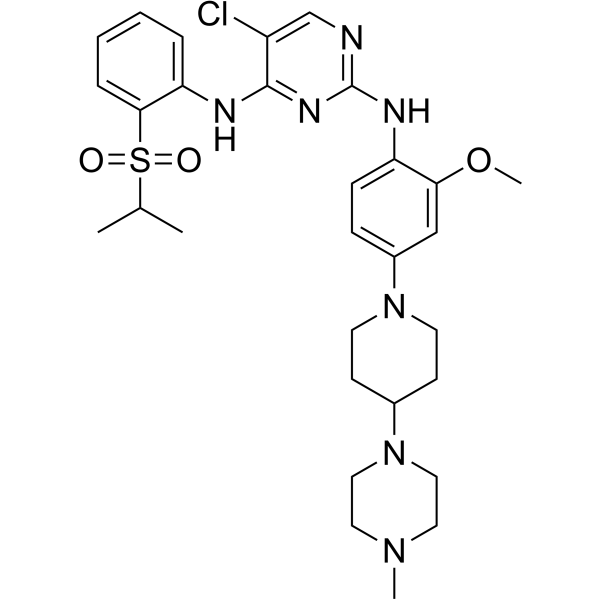


| Product Name | TAE684 (NVP-TAE684) |
| Price: | Inquiry |
| Catalog No.: | CN00362 |
| CAS No.: | 761439-42-3 |
| Molecular Formula: | C30H40ClN7O3S |
| Molecular Weight: | 614.2 g/mol |
| Purity: | >=98% |
| Type of Compound: | Alkaloids |
| Physical Desc.: | Powder |
| Source: | |
| Solvent: | Chloroform, Dichloromethane, Ethyl Acetate, DMSO, Acetone, etc. |
| SMILES: | COc1cc(ccc1Nc1ncc(c(n1)Nc1ccccc1S(=O)(=O)C(C)C)Cl)N1CCC(CC1)N1CCN(CC1)C |
| Contact us | |
|---|---|
| First Name: | |
| Last Name: | |
| E-mail: | |
| Question: | |
| Description | NVP-TAE 684 is a highly potent and selective ALK inhibitor, which blocks the growth of ALCL-derived and ALK-dependent cell lines with IC50 values between 2 and 10 nM. |
| In Vitro | TAE684 inhibits the proliferation of Ba/F3 NPM-ALK cells with an ICsub>50 of 3 nM, without affecting the survival of parental Ba/F3 cells at concentrations up to 1 μM. TAE684 inhibits STAT3 and STAT5 phosphorylation in a dose-dependent manner in both Ba/F3 NPM-ALK and Karpas-299 cells. TAE684 induces apoptosis and G1 phase arrest in NPM-ALK-expressing Ba/F3 cells and ALCL patient cell lines[1]. NVP-TAE684 markedly reduces cell survival in both sensitive H3122 and H3122 CR cells, but has little to no effect on the viability of other, non-ALK-dependent cancer cell lines. NVP-TAE684 treatment of H3122 CR cells suppresses phosphorylation of ALK, AKT, and ERK and induces marked apoptosis. TAE684 potently suppresses the survival of Ba/F3 cells expressing the EML4-ALK L1196M mutant[2]. Neurite outgrowth induced by expression of the mALKR1279Q mutant is completely inhibited at 30 nM NVP-TAE684, which is comparable with the response seen with activated wt mALK[3]. |
| In Vivo | NVP-TAE684 suppresses lymphomagenesis in two independent models of ALK-positive ALCL and induces regression of established Karpas-299 lymphomas. TAE684 displays appreciable bioavailability and half-life in vivo. TAE684 (1, 3, and 10 mg/kg. p.o.) significantly delays in lymphoma development and shows 100- to 1,000-fold reduction in luminescence signal. The TAE684- (10 mg/kg) treated group appeares healthy and does not display any signs of compound- or disease-related toxicity[1]. |
| Cell Assay | Luciferase-expressing Karpas-299, SU-DHL-1, and Ba/F3 cells and transformed Ba/F3 stably expressing NPM-ALK, BCR-ABL, or TEL-kinase fusion constructs are plated in 384-well plates (25,000 cells per well) and incubated with serial dilutions of TAE684 or DMSO for 2-3 days. Luciferase expression is used as a measure of cell proliferation/survival and is evaluated with the Bright-Glo Luciferase Assay System. ICsub>50 values are generated by using XLFit software. |
| Animal Admin | For in vivo compound efficacy studies, treatment is initiated 72 h after tail vein injection of 1×106 Karpas-299-, Ba/F3 NPM-ALK- or BCR-ABL-expressing cells into female Fox Chase SCIDBeige mice. Mice (n=10 per group) are administered either TAE684 resuspended in 10% 1-methyl-2-pyrrolidinone/90% PEG 300 solution at 1, 3, and 10 mg/kg once daily for 3 weeks or the vehicle solution at the same dosing schedule. Disease progression and compound efficacy is monitored weekly with bioluminescence imaging. To determine the efficacy of TAE684 on established disease, dosing is initiated on day 12, at which time the disease confirmed to be widespread by bioluminescence imaging. For analysis of downstream molecular effects in vivo, mice with established lymphomas are administered vehicle solution or TAE684 (10 mg/kg) for 3 days. At the end of treatment, mice are killed, and lymph nodes are extracted for immunoblotting and histological analysis. |
| Density | 1.3±0.1 g/cm3 |
| Boiling Point | 791.0±70.0 °C at 760 mmHg |
| Flash Point | 432.2±35.7 °C |
| Exact Mass | 613.260193 |
| PSA | 111.31000 |
| LogP | 2.71 |
| Vapour Pressure | 0.0±2.8 mmHg at 25°C |
| Storage condition | -20°C |